NOVAFIM - Escorts Heart Institute Angiographic Study
Agarwal P 1 , Bhandari S, Subramanyam K, Kapoor R, Kumar P, Chugh S, Shahi M, Singh B, Mathur A, Kasliwal R, Kler Ts, Trehan N.
Escorts Delhi .Indian Heart Journal 2006
Number of patients enrolled – 120
Inclusion Criteria
- De-Novo Lesions
- Multi-vessel disease
- Vessel Diameter 2.5-3.5mm
- Target Lesion <30mm
- Thrombotic lesions occupying
Exclusion Criteria
- Ongoing AMI
- Post CABG patients
- Unprotected left main artery
- Involving side branch of –>2mm diameter
Results
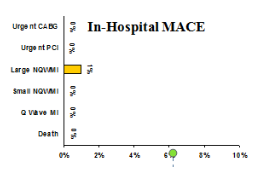
- In Hospital MACE
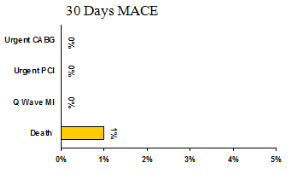
- 30 Days MACE
NovaFim - 6 Months QCA Results
- Total patients enrolled – 120
- Total stents deployed – 168
- Avgerage stents / patients – 1.40
- No. of angiographic follow ups done – 97(81%)
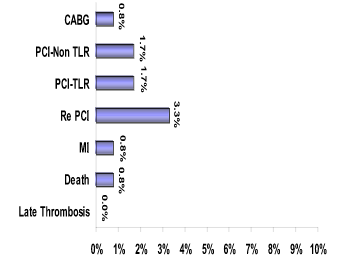
Primary Endpoint
- 100% successful deployment
- In-Hospital MACE - 1 patient CABG & MI(wire induced)
- 30 day MACE - 1 Death
Secondary End points
TLR 1.66%, 1 Death, 1 MI (occurred within 30 days)
Conclusion
Pronova stent is efficious and safe with low MACE rates.


